MECHANISM OF ACTION
Aldurazyme® targets IDUA enzyme deficiency—the cause of ongoing damage in MPS I1,2
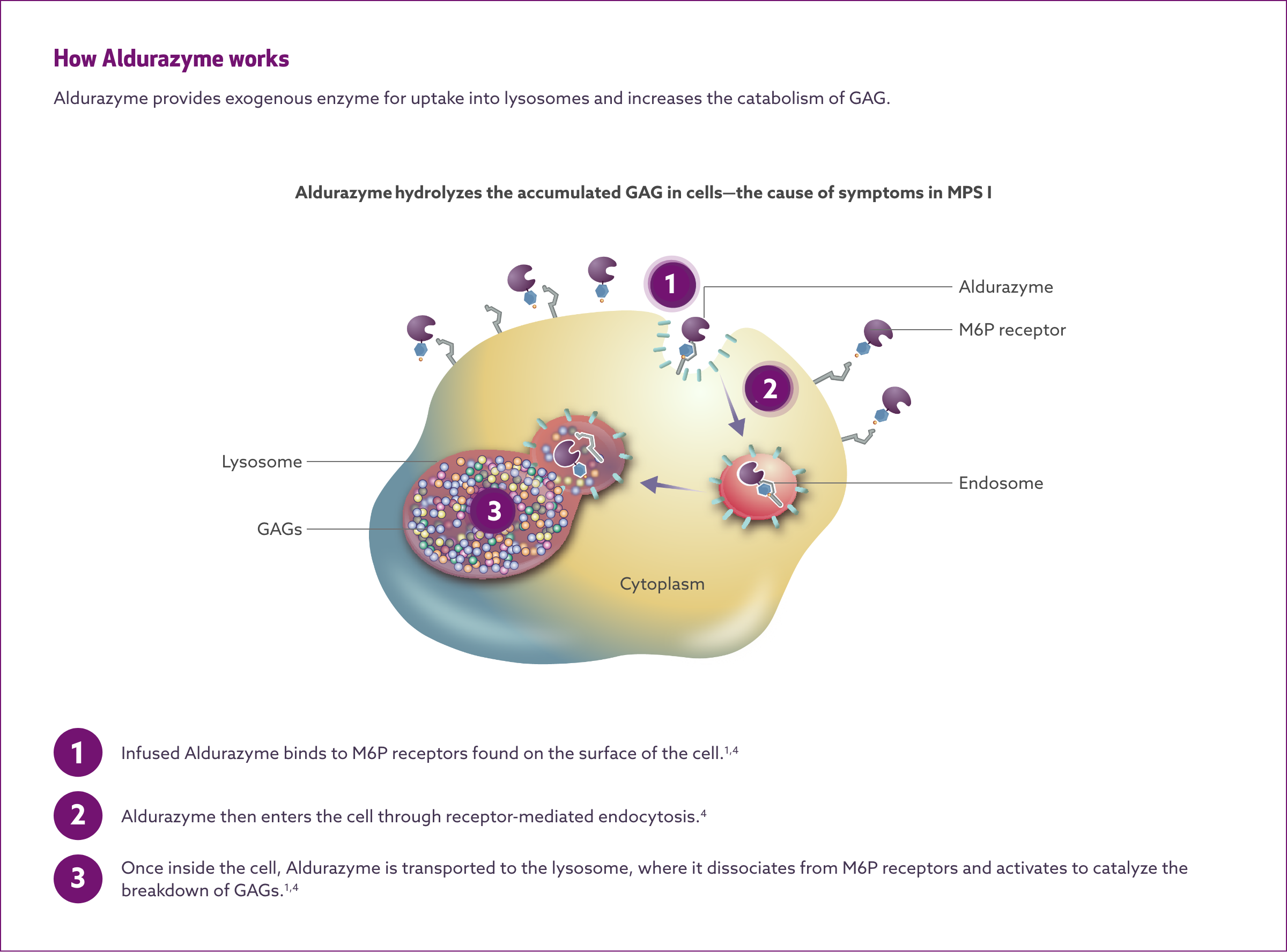
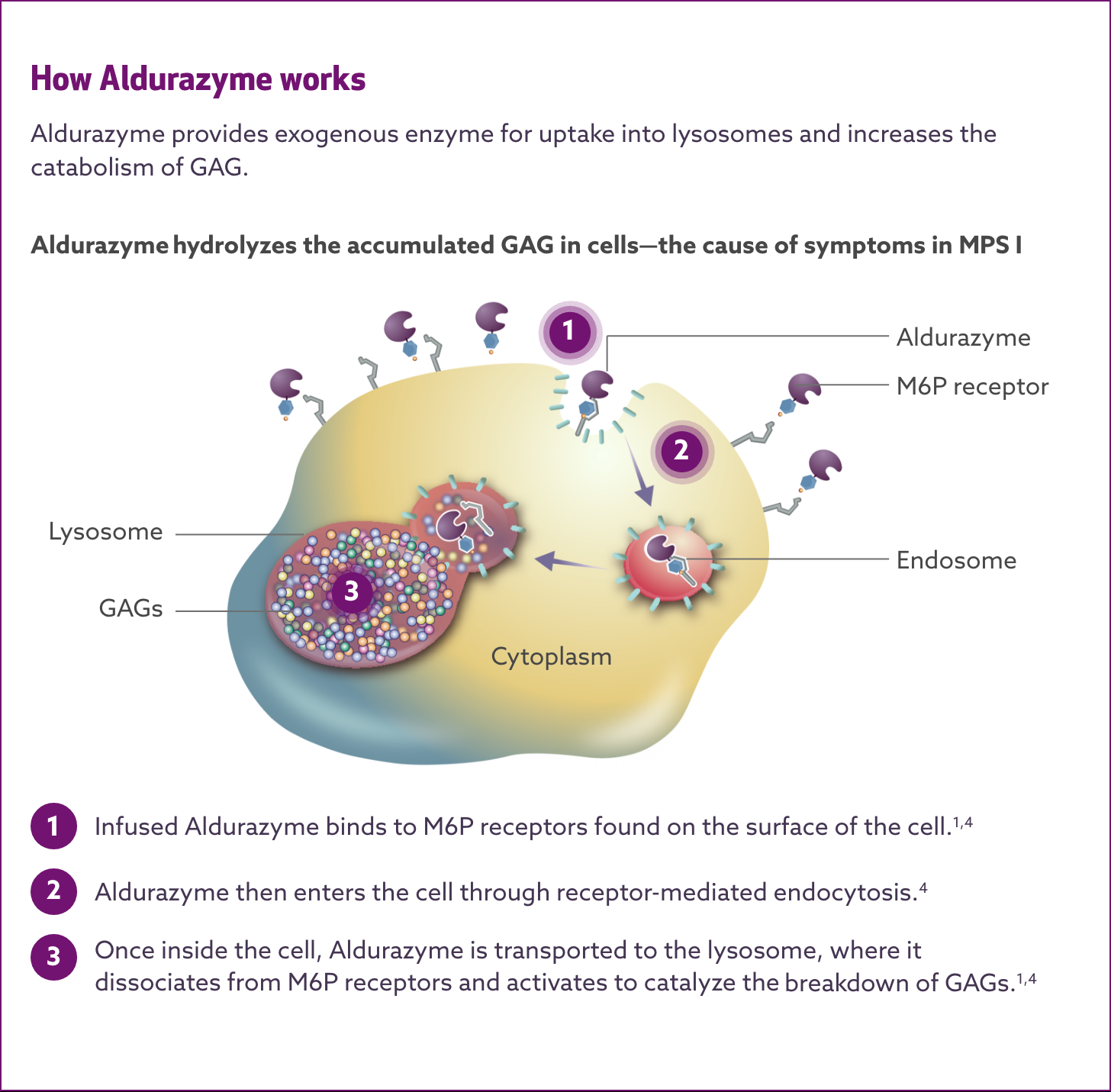
Aldurazyme substitutes the naturally occurring enzyme α-L-iduronidase, which is deficient or absent in MPS I patients.1-3
Aldurazyme uptake by cells into lysosomes is most likely mediated by the mannose-6-phosphate (M6P)-terminated oligosaccharides chains of laronidase binding to specific M6P receptors. Aldurazyme provides exogenous enzyme for uptake into lysosomes and increases the catabolism of glycosaminoglycan (GAG).
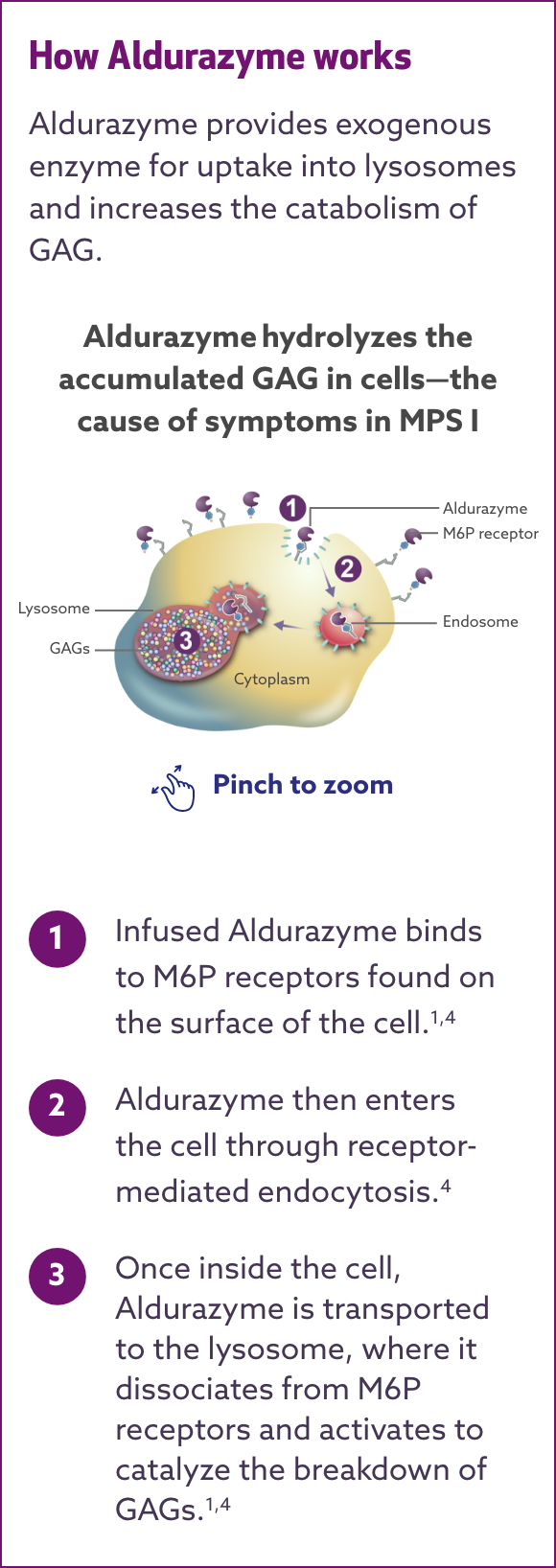
GAG = glycosaminoglycan; IDUA = α-L-iduronidase; M6P = mannose 6-phosphate; MPS I = mucopolysaccharidosis type I.



Get started with Aldurazyme – Thinking about prescribing Aldurazyme?
EXPLORE DOSING AND ADMINISTRATIONCLINICAL TRIAL RESULTS
Aldurazyme® showed improved respiratory function and walking distance in patients with MPS I
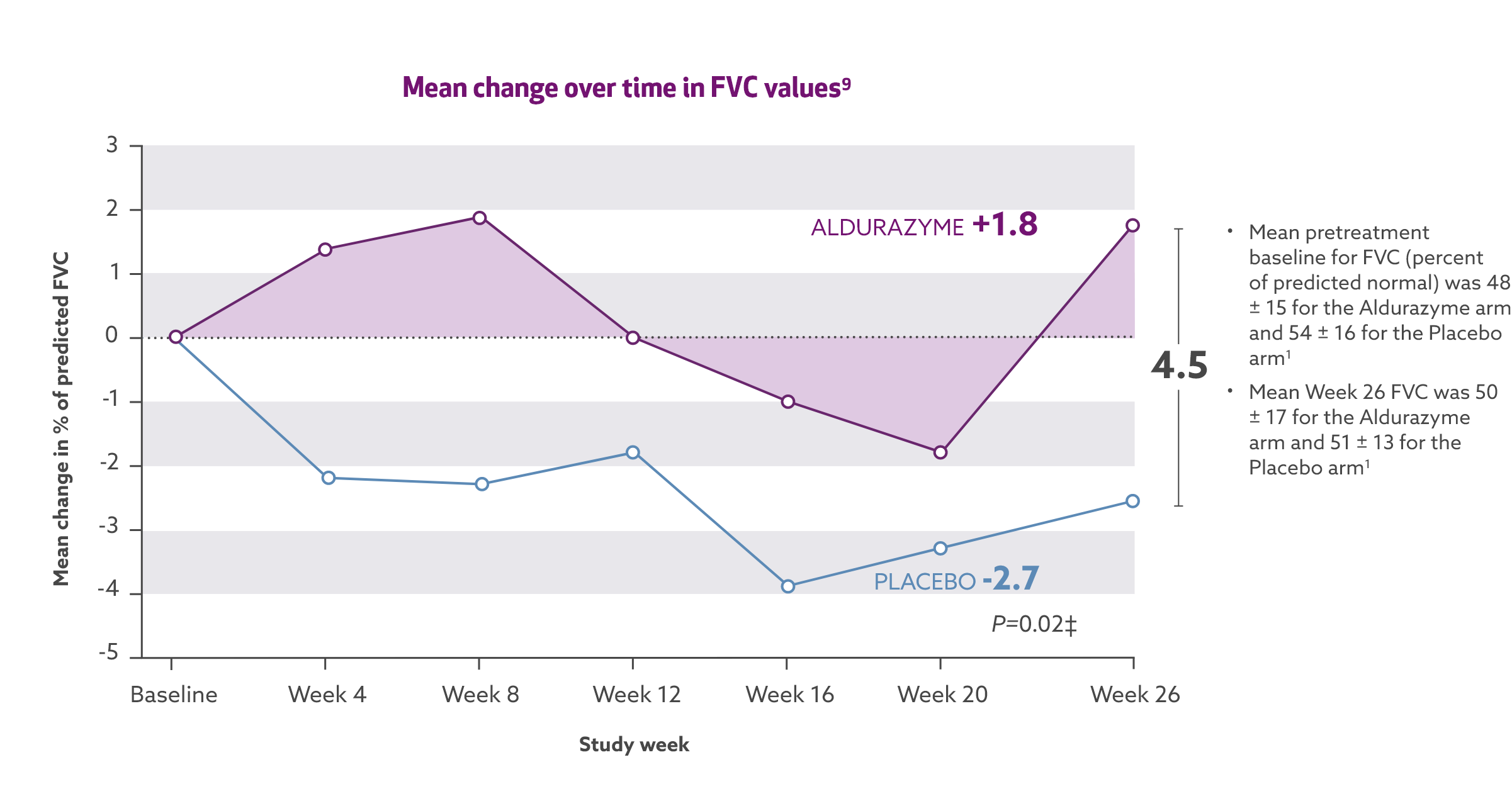
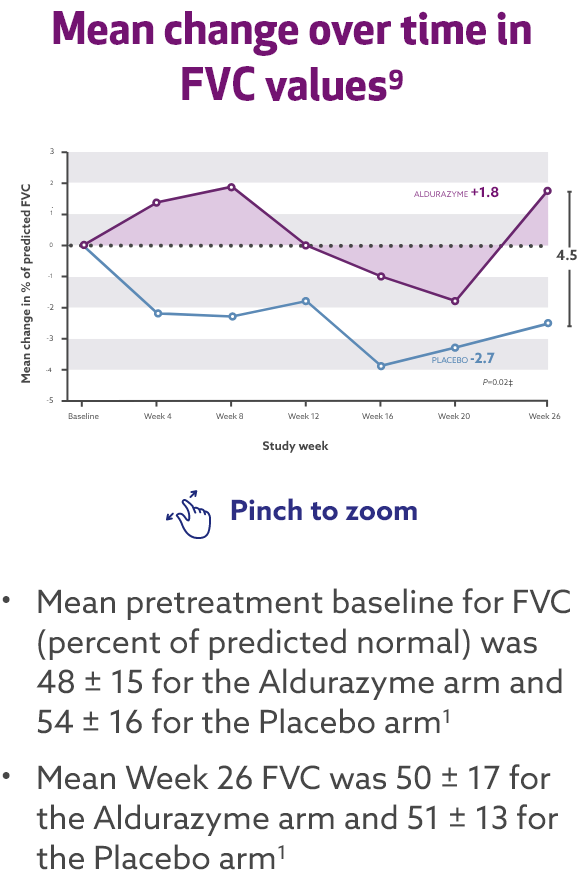
Aldurazyme improved pulmonary function (FVC) in patients with MPS I1*†
In the 26-week, randomized, double-blind, placebo-controlled study, patients treated with Aldurazyme (n=22) experienced a mean 4-point increase in percent predicted forced vital capacity (FVC) compared with those receiving placebo (n=23) (median difference 2.0 [95% CI:0.4, 7] p=0.02).1
Study 1 was a randomized, double-blind, placebo-controlled study in 45 patients with MPS I, aged 6-43 years, including 1 patient with the Hurler form, 37 patients with Hurler-Scheie form, and 7 patients with Scheie form of MPS I. All patients had a baseline percent predicted FVC less than or equal to 77%. Patients received Aldurazyme at 0.58 mg/kg of body weight once weekly or placebo once weekly for 26 weeks. All patients were treated with antipyretics and antihistamines prior to each infusion. The primary efficacy outcome assessments were percent predicted FVC and distance walked in 6 minutes (6-MWT).1

*The risks and benefits of treating mildly affected patients with the Scheie form have not been established.
†Aldurazyme has not been evaluated for its effects on the central nervous system manifestations of the disorder.
‡Wilcox Rank Sum Test on median of difference.
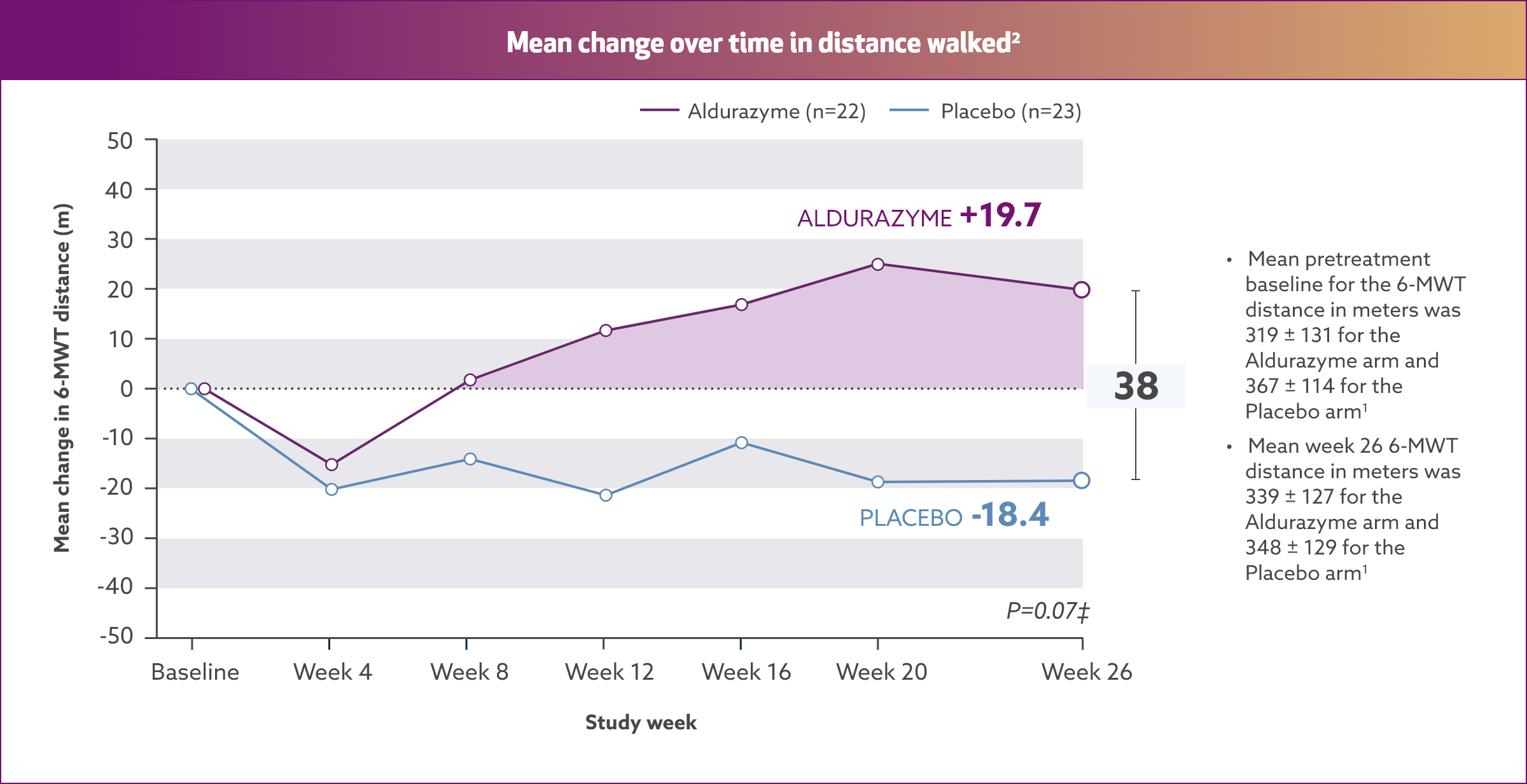
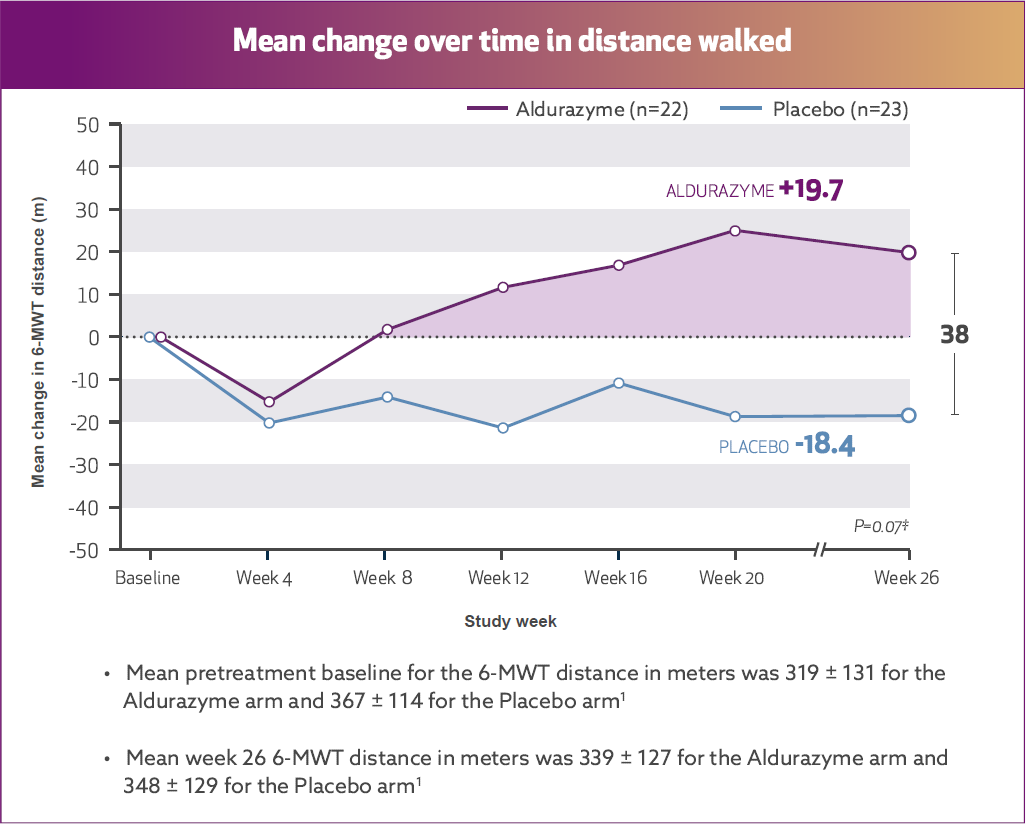
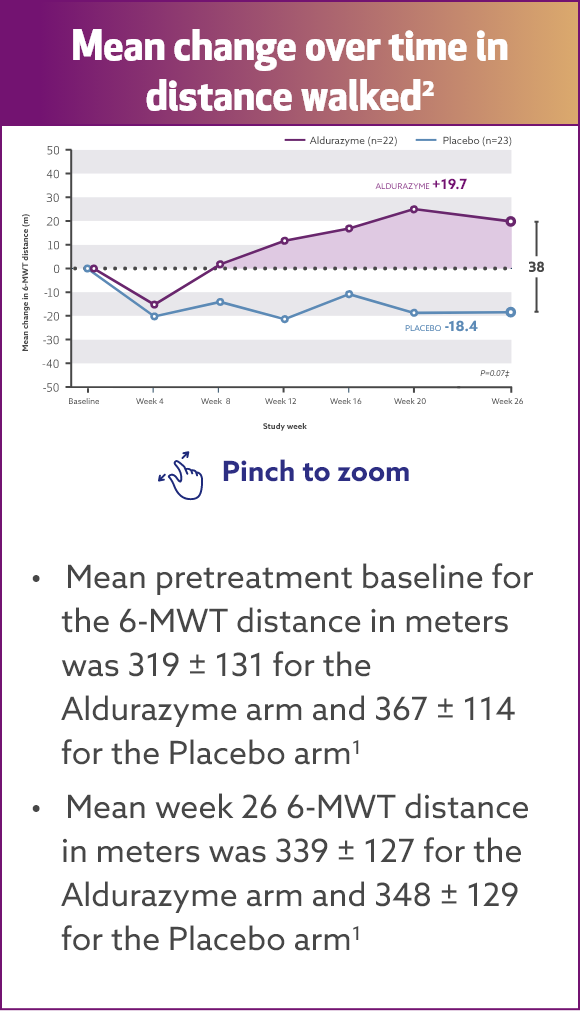
Aldurazyme improved walking distance (6-MWT) in patients with MPS I1*†
In the 26-week, randomized, double-blind, placebo-controlled study, patients treated with Aldurazyme (n=22) showed a mean 38-m increase in distance walked (as measured by the 6-MWT) compared with those receiving placebo (n=23) (median difference 39 [95% CI: -2.0, 79.0] p=0.07).1,2
Study 1 was a randomized, double-blind, placebo-controlled study in 45 patients with MPS I, ages 6 to 43 years old, including 1 patient with the Hurler form, 37 patients with Hurler-Scheie form, and 7 patients with Scheie form of MPS I. All patients had a baseline percent predicted forced vital capacity (FVC) less than or equal to 77%. Patients received Aldurazyme at 0.58 mg/kg of body weight once weekly or placebo once weekly for 26 weeks. All patients were treated with antipyretics and antihistamines prior to each infusion. The primary efficacy outcome assessments were percent predicted FVC and distance walked in 6 minutes (6-MWT).
*The risks and benefits of treating mildly affected patients with the Scheie form have not been established.
†Aldurazyme has not been evaluated for its effects on the central nervous system manifestations of the disorder.
‡Wilcox Rank Sum Test on median of difference.
Study 2 was a 182-week, open-label, uncontrolled extension study of all 45 patients who completed Study 1. Patients received Aldurazyme at 0.58 mg/kg body weight once weekly.
For patients treated with Aldurazyme, the mean increase in the 6‑MWT distance was maintained for an additional 182 weeks through the completion of Study 2.1
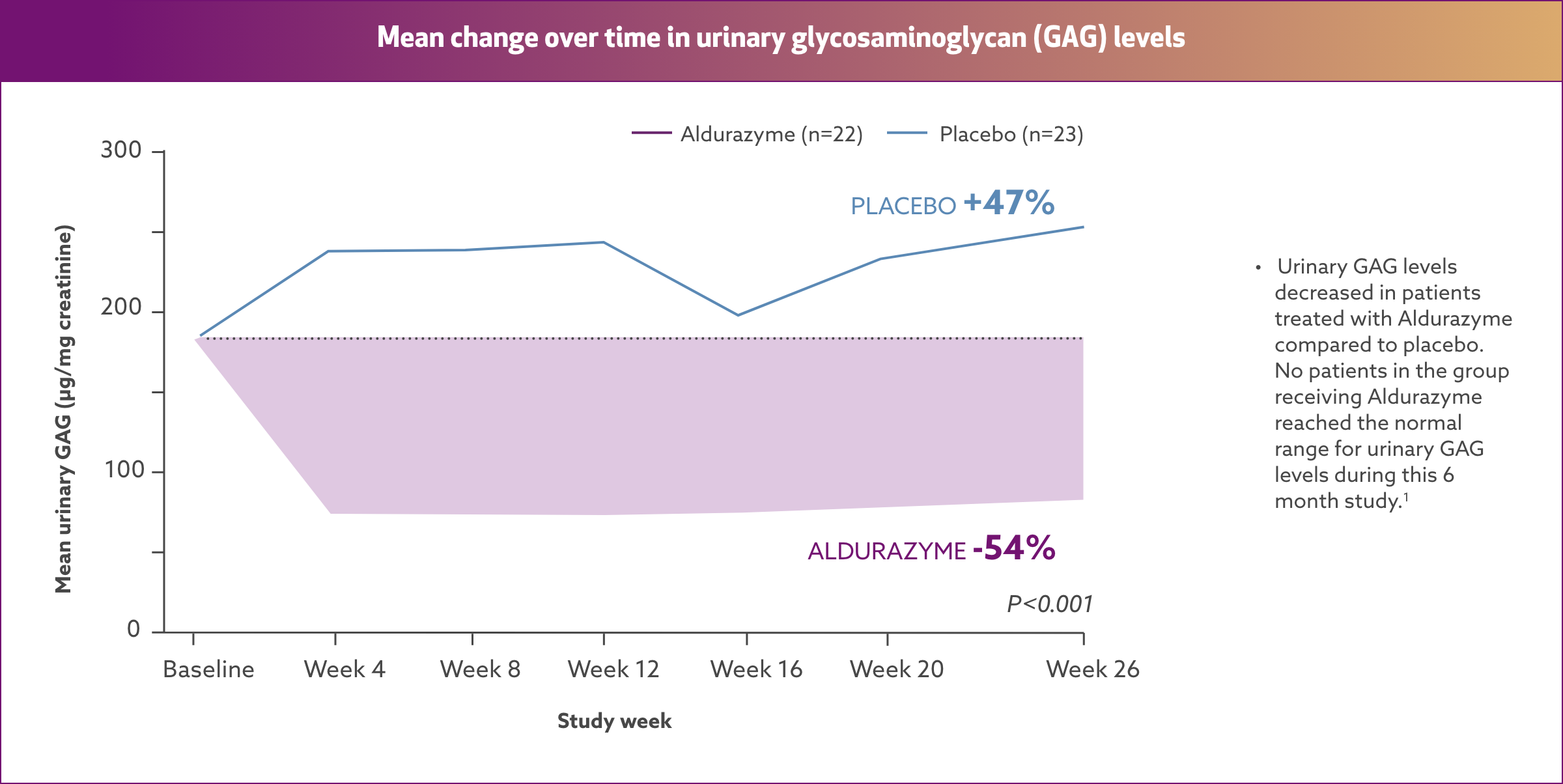
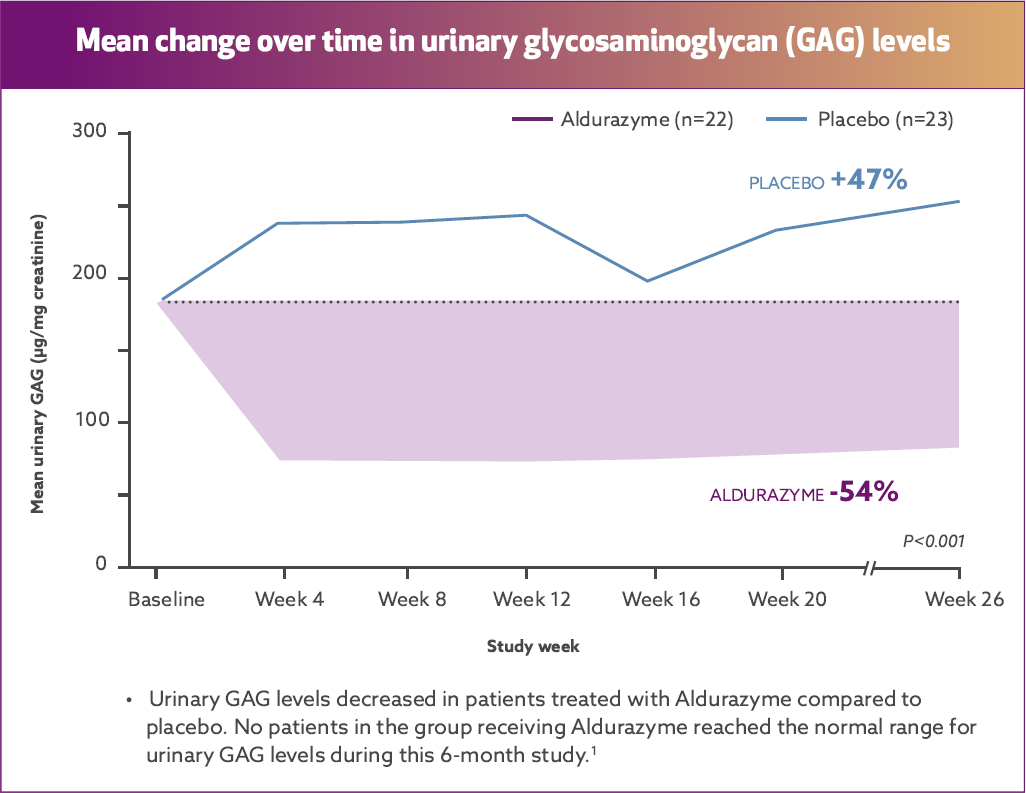
Aldurazyme decreased urinary glycosaminoglycan (GAG) level by 54%2*†
Results from a randomized, double‑blind, placebo-controlled study of 45 MPS I patients (aged 6‑43 years) measuring mean change over time in urinary GAG level (n=22 and 23 for the placebo group and n=21 and 22 for the Aldurazyme group, respectively, at baseline and week 26).
See trial design for Study 1 above.
See trial design for Study 2 above.
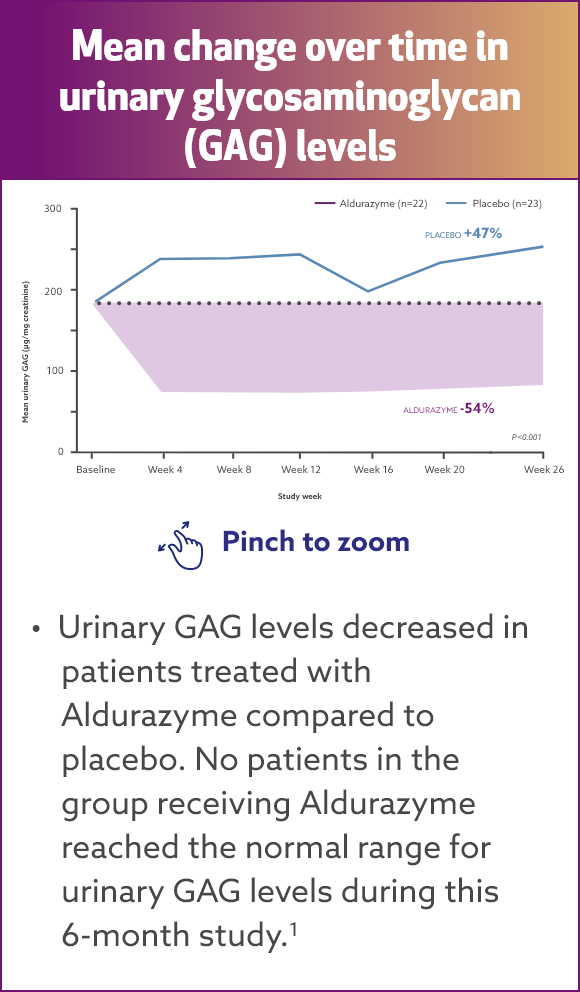
*The risks and benefits of treating mildly affected patients with the Scheie form have not been established.
†Aldurazyme has not been evaluated for its effects on the central nervous system manifestations of the disorder. Evaluations of bioactivity were changes in liver
size and urinary GAG levels.
At the end of Study 2, the decrease in mean urinary GAG was similar to the decrease in urinary GAG reported in the Aldurazyme-treated patients at the end of Study 1.1
The relationship of urinary GAG to other measures of clinical response has not been established.1
6-MWT = six-minute walk test; FVC = forced vital capacity; GAG = glycosaminoglycan; MPS I = mucopolysaccharidosis type I.
SAFETY PROFILE
Warnings and Precautions
Hypersensitivity Reactions Including Anaphylaxis: See Boxed WARNING.
- Pre-existing upper airway obstruction may contribute to the severity of some reactions. Consider premedicating patients with antihistamines, with or without antipyretics. Because of the potential for recurrent reactions, some patients who experience initial severe reactions may require prolonged observation.
- Consider risks and benefits of re-administering ALDURAZYME following severe hypersensitivity reactions. Patients may be rechallenged using slower infusion rates which may be increased if tolerated to reach the recommended rate. If a mild or moderate hypersensitivity reaction occurs, consider temporarily holding the infusion or slowing the infusion rate.
Acute Respiratory Complications Associated with Administration: See Boxed WARNING.
- Patients with an acute febrile or respiratory illness may be at greater risk for infusion reactions. Consider the patient’s clinical status prior to administration of ALDURAZYME and consider delaying the infusion.
- Evaluation of airway patency should be considered prior to initiating ALDURAZYME. Patients using supplemental oxygen or continuous positive airway pressure (CPAP) during sleep should have these treatments readily available during infusion in the event of an infusion reaction or extreme drowsiness/sleep induced by antihistamine use.
Acute Cardiorespiratory Failure:
- Use caution when administering ALDURAZYME to patients susceptible to fluid overload, or with an acute underlying respiratory illness or compromised cardiac and/or respiratory function for whom fluid restriction is indicated. Consider a decreased total infusion volume and infusion rate when administering ALDURAZYME to these patients.
Infusion-Associated Reactions:
- ALDURAZYME may cause infusion-associated reactions (IARs). Consider pre-medicating with antihistamines, with or without antipyretics, however IARs may still occur in patients after receiving pre-medication. Discontinue immediately or adjust the infusion rate based on the severity of the reaction.
For additional details about these Warnings and Precautions, see the Important Safety Information below.
Aldurazyme® has demonstrated a safety profile*1
Serious adverse reactions reported with Aldurazyme treatment during clinical trials were anaphylactic and hypersensitivity reactions
The most common adverse reactions were infusion reactions. The frequency of infusion reactions decreased over time with continued use of Aldurazyme, and the majority of reactions were classified as being mild to moderate in severity.
In a clinical trial of patients 6 years and older, infusion reactions were reported in 32% (7 of 22) of Aldurazyme (laronidase)-treated patients.¹ The most commonly reported infusion reactions were flushing, pyrexia, headache, and rash. Flushing occurred in 5 patients (23%) receiving Aldurazyme; the other reactions were less frequent.
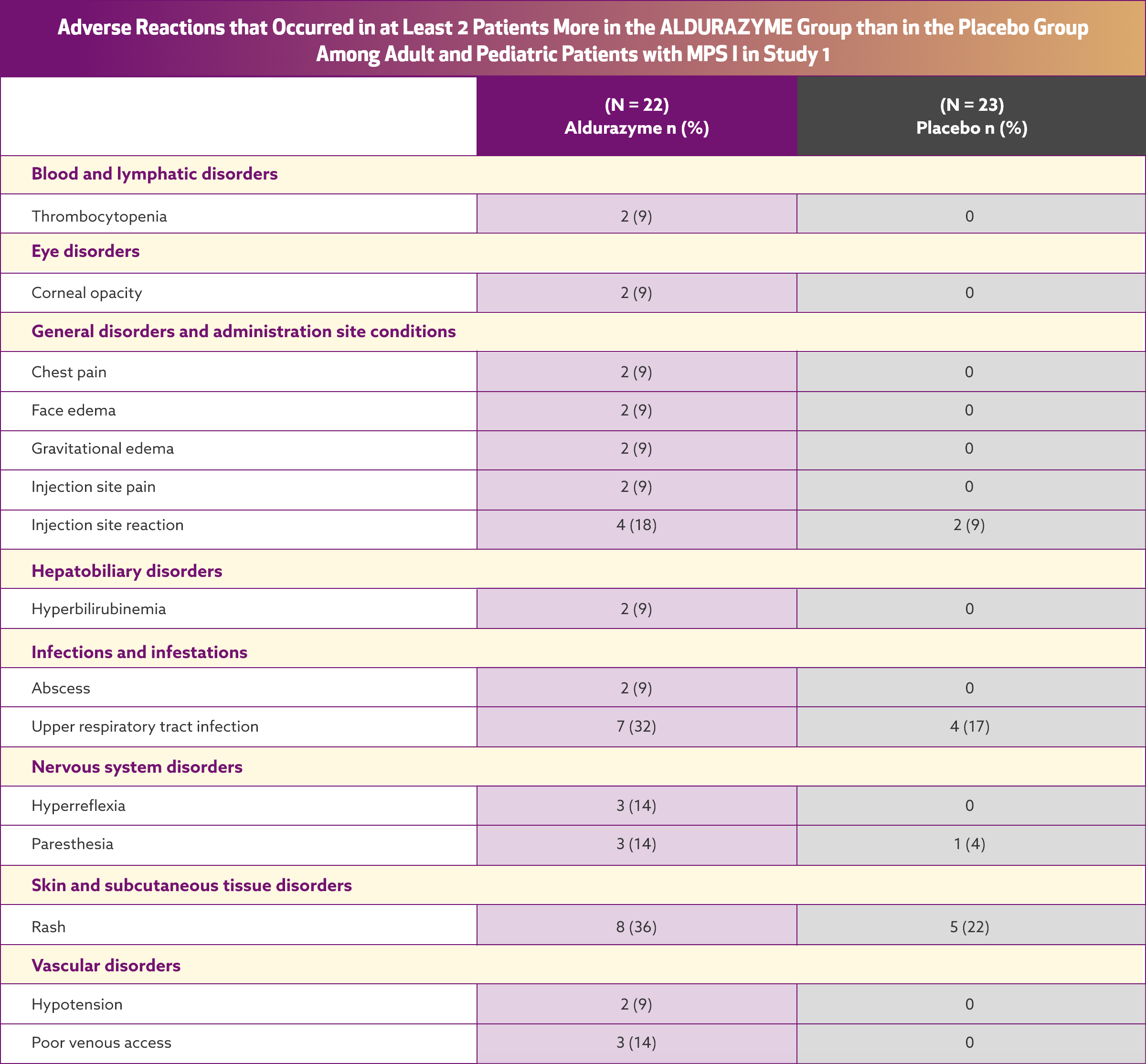
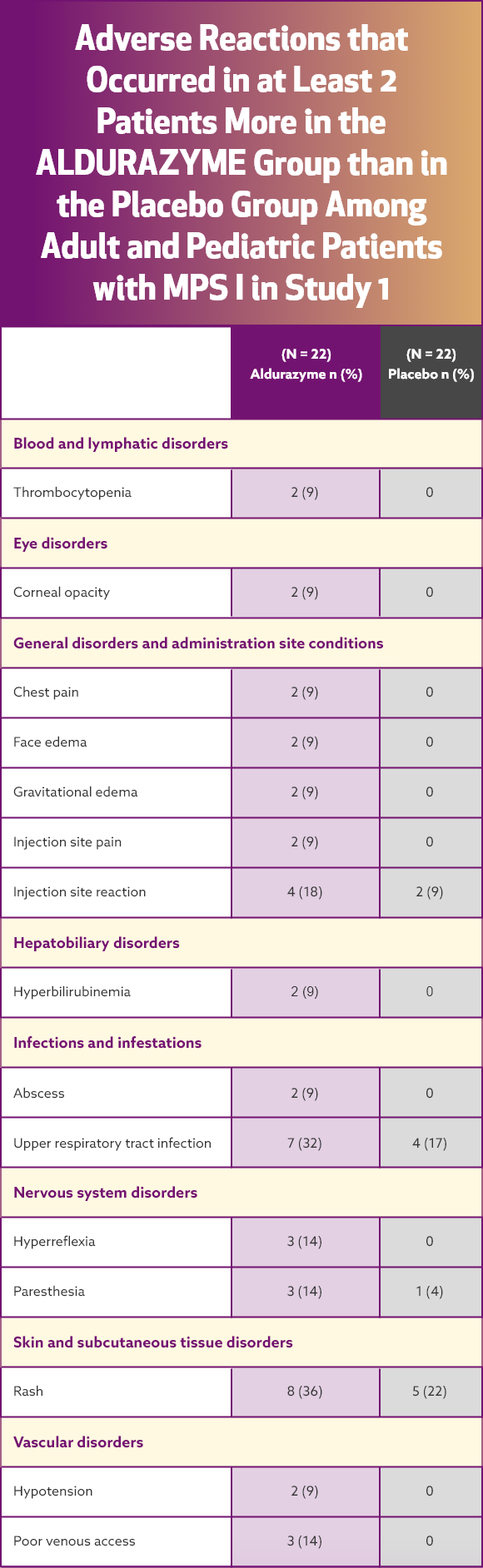
AE = adverse event; IAR = infusion-associated reaction.
References: 1. ALDURAZYME [prescribing information]. Cambridge, MA: Genzyme Corporation. 2. Wraith JE, Clarke LA, Beck M, et al. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-Liduronidase (laronidase). J Pediatr. 2004;144(5):581-588. 3. de Ru MH, Boelens JJ, Das AM, et al. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J Rare Dis. 2011;6(55):1-9. 4. Wraith EJ, Hopwood JJ, Fuller M, Meikle PJ, Brooks DA. Laronidase treatment of mucopolysaccharidosis I. BioDrugs. 2005;19(1):1-7. 5. Data on File. Genzyme Corporation.