WHAT DOES ALDURAZYME DO?
Outcomes of clinical studies1,2
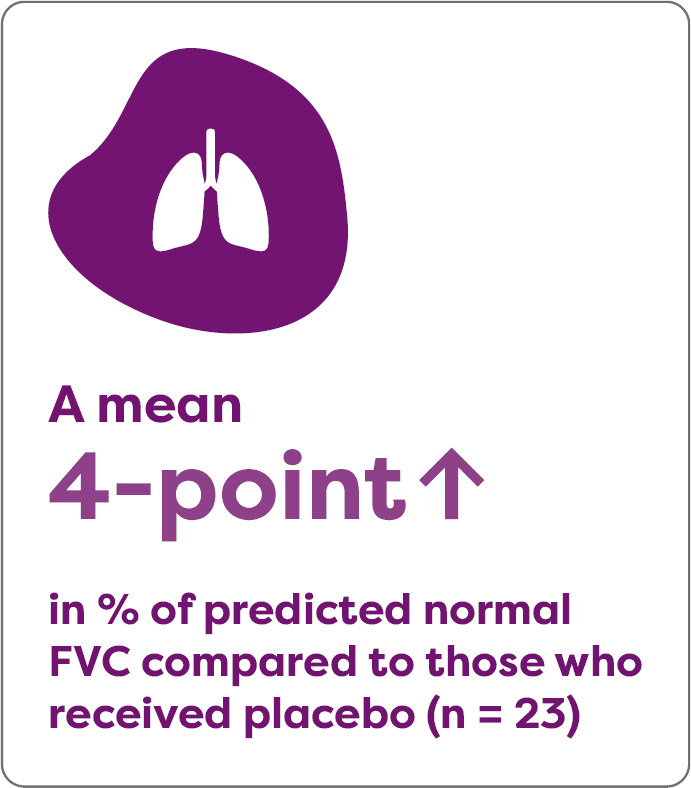
Aldurazyme improved pulmonary function in patients with MPS I
- Study 1 was double-blind, placebo-controlled, neither the HCPs nor the patients knew what the treatment was, either Aldurazyme or placebo, during the study. 37 patients with the Hurler-Scheie form, and 7 patients with the Scheie form of MPS I.
- At the beginning of the study, all patients had a percent predicted forced vital capacity (FVC) of ≤77%.
- Patients received Aldurazyme at 0.58 mg/kg of body weight once weekly or placebo once weekly for 26 weeks.
- All patients were treated with antipyretics and antihistamines prior to each infusion.
- The study measured FVC (forced vital capacity, which measures how much air you can breathe out in one forceful breath) and 6MWT (6-minute walk test, which measures the distance you can walk within that time frame).
In the 26-week study, 22 patients were treated with Aldurazyme and experienced



Study 2 was a 182-week, open-label, uncontrolled extension study of all 45 patients who completed Study 1. Patients received Aldurazyme at 0.58 mg/kg body weight once weekly. For patients treated with Aldurazyme, the mean increase in the 6-MWT distance was maintained for an additional 182 weeks through the completion of study 2.
Aldurazyme improved pulmonary function and walking distance in patients with MPS I
In the same 26-week study (study 1), urinary GAG levels decreased in patients treated with ALDURAZYME compared to patients treated with placebo. No patient in the group receiving ALDURAZYME reached the normal range for urinary GAG levels during this 6-month study.
Aldurazyme decreased urinary GAG level in patients with MPS I
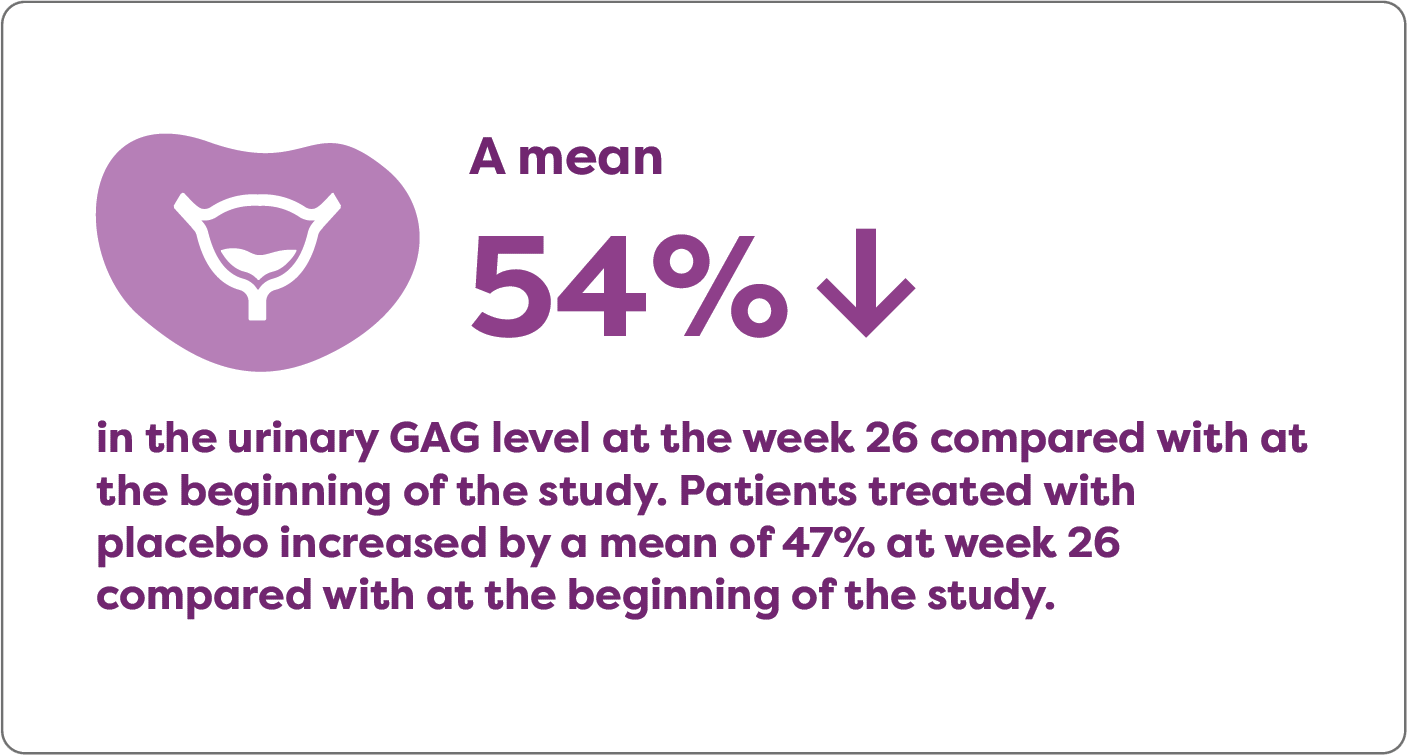
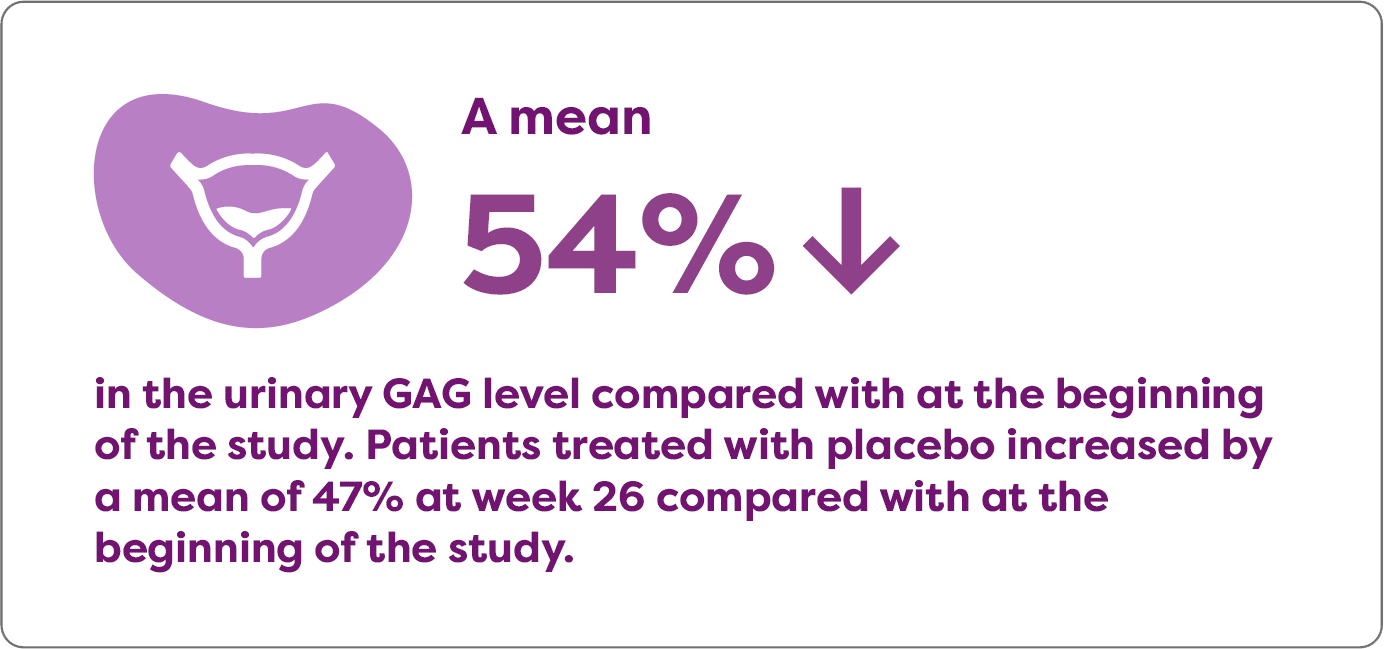

In a randomized, double-blind, placebo-controlled study that measured the mean change over time in urinary GAG levels
References: 1. ALDURAZYME. Prescribing Information. Cambridge, MA: Genzyme Corporation; 2020. 2. Wraith JE, Clarke LA, Beck M, et al. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase). J Pediatr. 2004;144(5):581-588.

